Top 4 eConsent Questions in Clinical Research: Forms & More
Por um escritor misterioso
Last updated 28 março 2025

eConsent In Clinical Trials Insights For Implementation During

eConsent: Sites and Sponsors Tout Benefits, Confront Obstacles
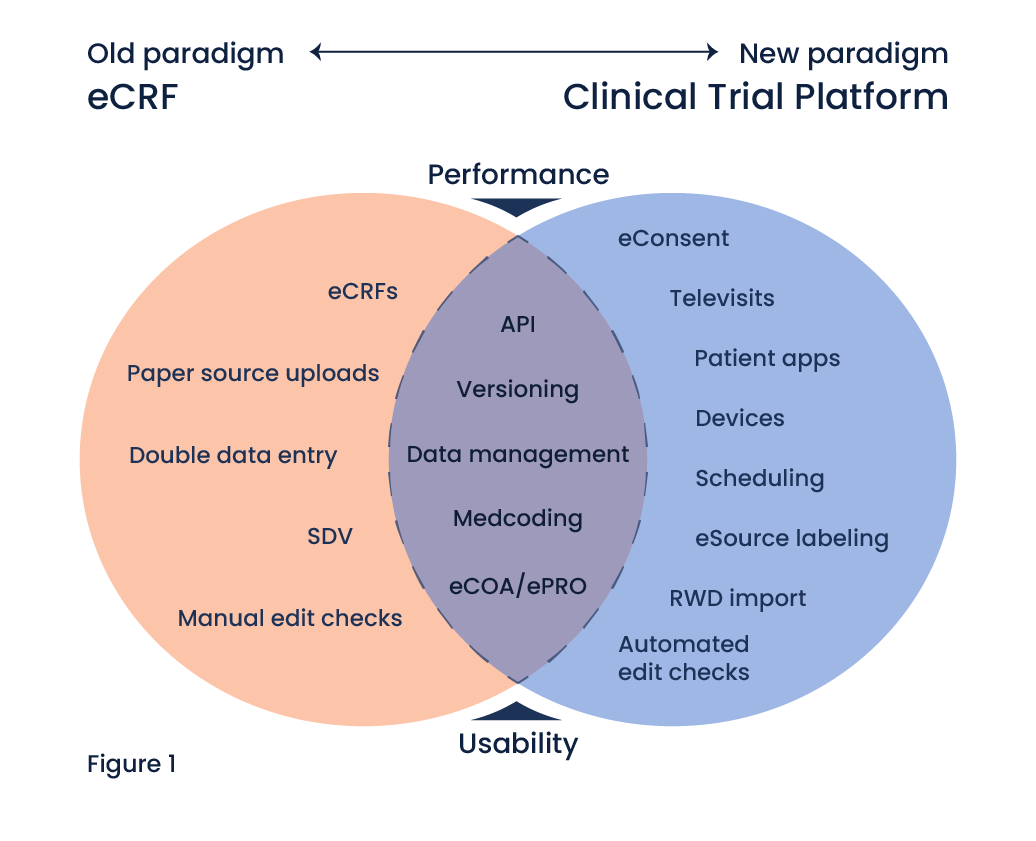
eCRF in Clinical Trials: Shifting to a Modern Research Paradigm
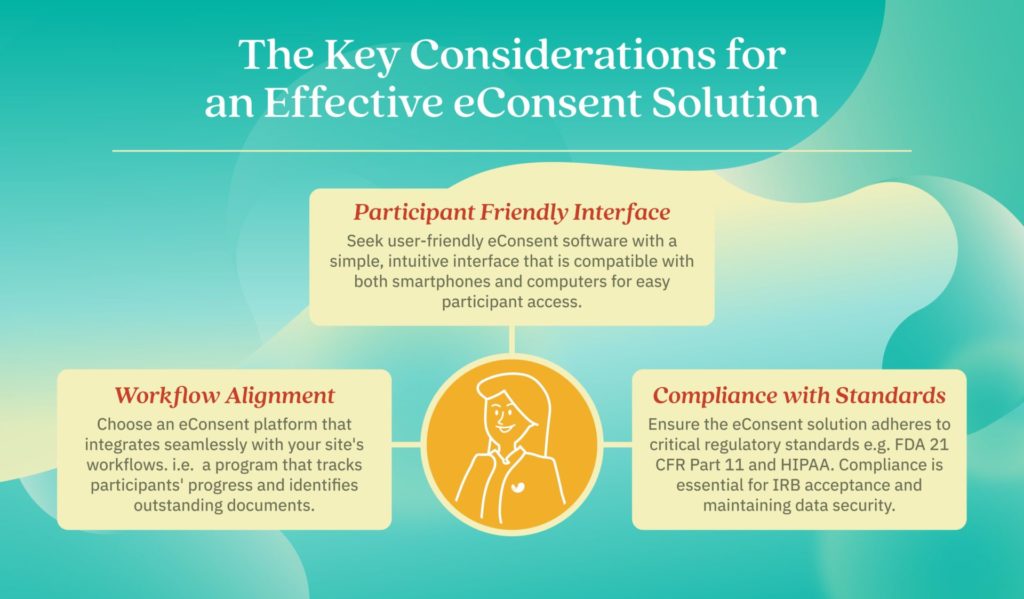
A Step-by-Step Guide to eConsent ICF (Informed Consent Form

E-Consent for Clinical Trials - Best Practices & Common Mistakes

How To Measure What Matters in Clinical Trials
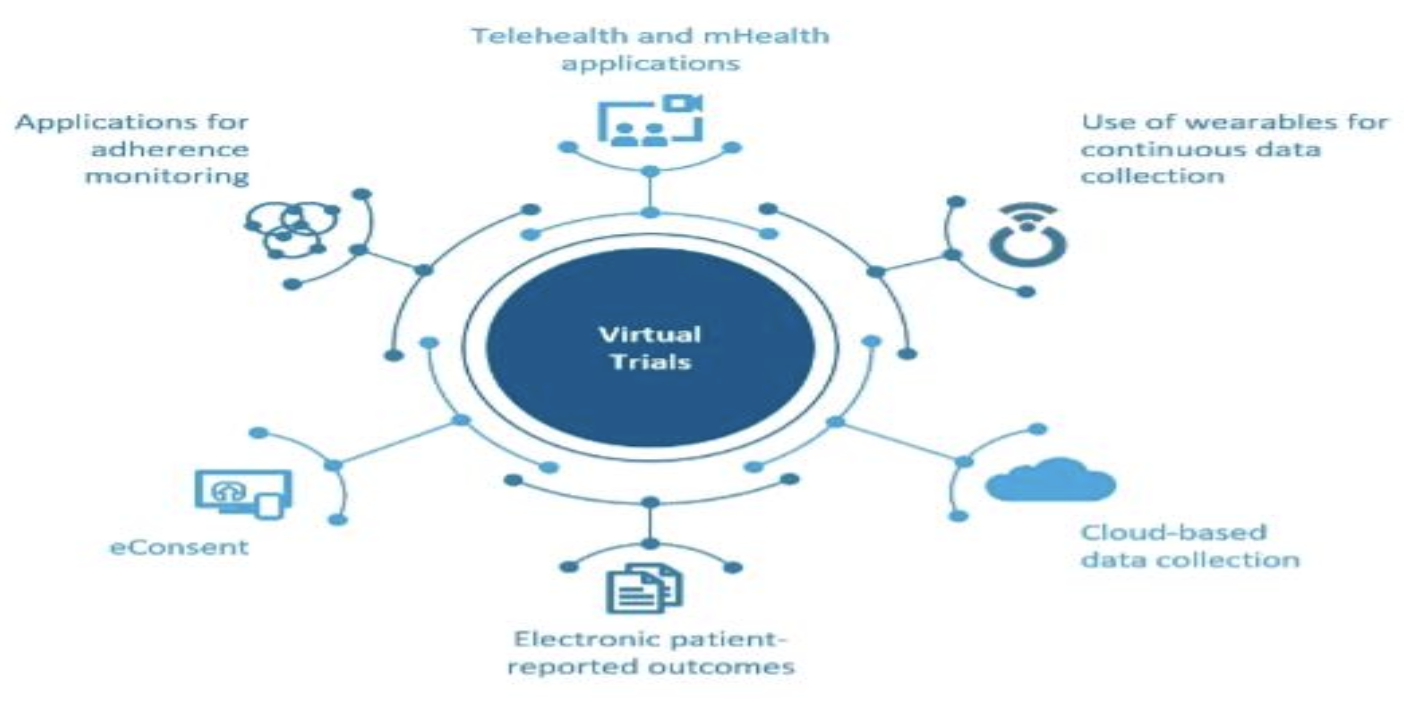
Current Professionals — Clinical Research Certification I Blog - CCRPS
Top 4 eConsent Questions in Clinical Research: Forms & More
How to Avoid the Top 5 Clinical Trial FDA Inspection Failures
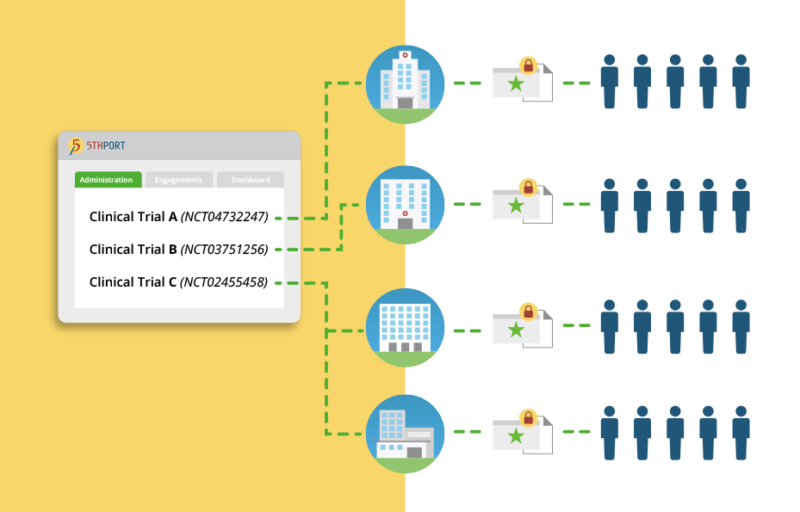
Clinical Solution, eConsent for Clinical Trials

SMART-TRIAL Releases New eConsent Add-On
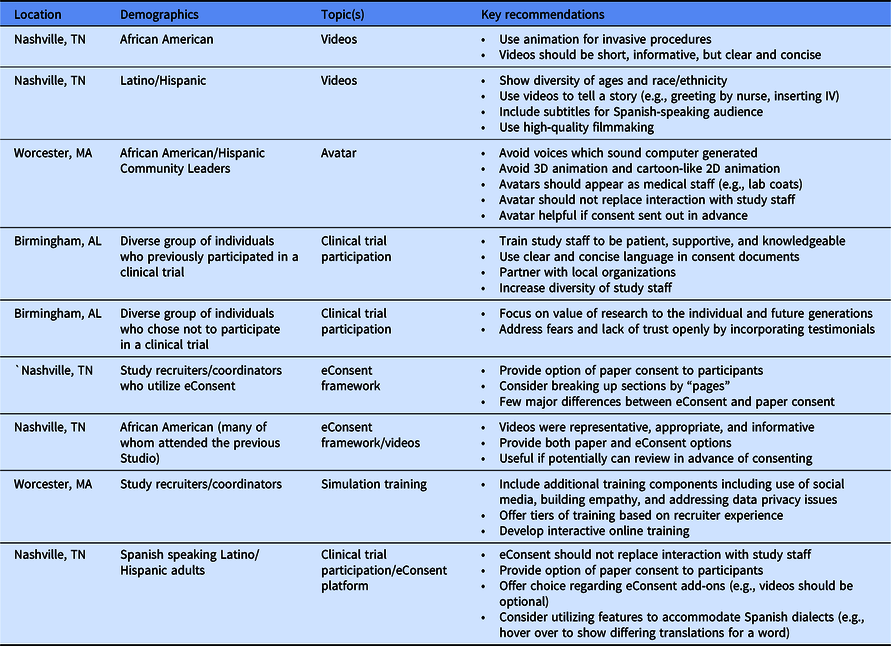
A REDCap-based model for electronic consent (eConsent): Moving

A REDCap-based model for electronic consent (eConsent): Moving
Recomendado para você
-
How do I upload images and GIFs? – Discord28 março 2025
-
 Heart Animated Sticker28 março 2025
Heart Animated Sticker28 março 2025 -
 MakeSweet: Create pictures and animations in 3D28 março 2025
MakeSweet: Create pictures and animations in 3D28 março 2025 -
 Make jigsaw puzzles video online28 março 2025
Make jigsaw puzzles video online28 março 2025 -
 Tabletop Smooth Heart Life Token28 março 2025
Tabletop Smooth Heart Life Token28 março 2025 -
 hearts - Free animated GIF - PicMix28 março 2025
hearts - Free animated GIF - PicMix28 março 2025 -
 What kind of boyfriend are you based on your zodiac sign?28 março 2025
What kind of boyfriend are you based on your zodiac sign?28 março 2025 -
 Job Search Accelerator Program28 março 2025
Job Search Accelerator Program28 março 2025 -
 A Hookup App for the Emotionally Mature28 março 2025
A Hookup App for the Emotionally Mature28 março 2025 -
:max_bytes(150000):strip_icc()/VWH-Julie-Bang-Dangerous-Heart-Rate-in-Children-Standard-7e6cd8ff50da4be2905a3946bf47a808.gif) What Is a Dangerous Heart Rate?28 março 2025
What Is a Dangerous Heart Rate?28 março 2025
você pode gostar
-
 MICROSOFT XBOX ONE S Console 500GB 1681 MICROSOFT XBOX ONE S 500GB VERY GOOD28 março 2025
MICROSOFT XBOX ONE S Console 500GB 1681 MICROSOFT XBOX ONE S 500GB VERY GOOD28 março 2025 -
 Ghost Of Tsushima 2: Answering ALL your questions about the sequel! : r/ghostoftsushima28 março 2025
Ghost Of Tsushima 2: Answering ALL your questions about the sequel! : r/ghostoftsushima28 março 2025 -
 Barbie – Como não amar?28 março 2025
Barbie – Como não amar?28 março 2025 -
/i.s3.glbimg.com/v1/AUTH_bc8228b6673f488aa253bbcb03c80ec5/internal_photos/bs/2021/y/v/wHhuPlSYWafQWDGLolGA/litten.png) Pokémon 25 anos: os melhores iniciais de cada geração da franquia28 março 2025
Pokémon 25 anos: os melhores iniciais de cada geração da franquia28 março 2025 -
 Confiança do empresário do comércio cai pela primeira vez no ano28 março 2025
Confiança do empresário do comércio cai pela primeira vez no ano28 março 2025 -
The 25 Best Mekakucity Actors Quotes (With Images)28 março 2025
-
 Vikings: Valhalla Cast and Character Guide: Who's Who in the Netflix Show28 março 2025
Vikings: Valhalla Cast and Character Guide: Who's Who in the Netflix Show28 março 2025 -
 Large Face Plastic-Coated Playing Cards (Set of 12)28 março 2025
Large Face Plastic-Coated Playing Cards (Set of 12)28 março 2025 -
 Watch Dogs: Legion Review: Reflective But Unthoughtful Resistance28 março 2025
Watch Dogs: Legion Review: Reflective But Unthoughtful Resistance28 março 2025 -
![Shadow Fruit in Blox Fruits Info, Guide, Combos [UPDATE 20.1] ⭐](https://static.wikia.nocookie.net/roblox-blox-piece/images/d/d2/Corvus_Torment.gif) Shadow Fruit in Blox Fruits Info, Guide, Combos [UPDATE 20.1] ⭐28 março 2025
Shadow Fruit in Blox Fruits Info, Guide, Combos [UPDATE 20.1] ⭐28 março 2025
