Fluorescent Single-Stranded DNA Binding Protein as a Probe for Sensitive, Real-Time Assays of Helicase Activity: Biophysical Journal
Por um escritor misterioso
Last updated 31 março 2025


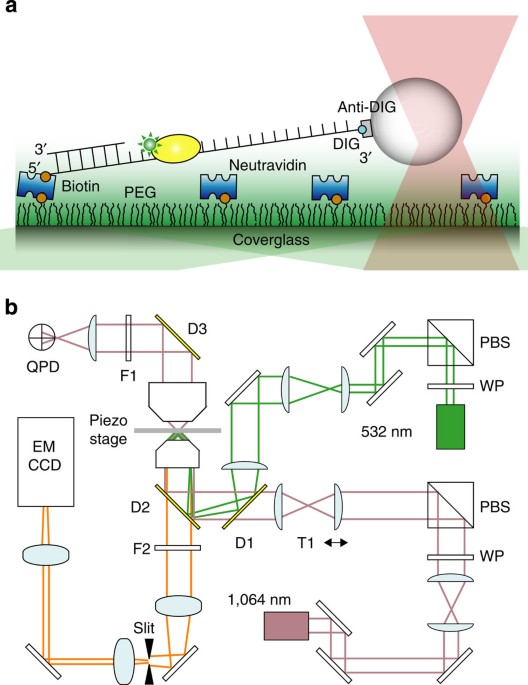
DNA replication machinery: Insights from in vitro single-molecule approaches - Computational and Structural Biotechnology Journal
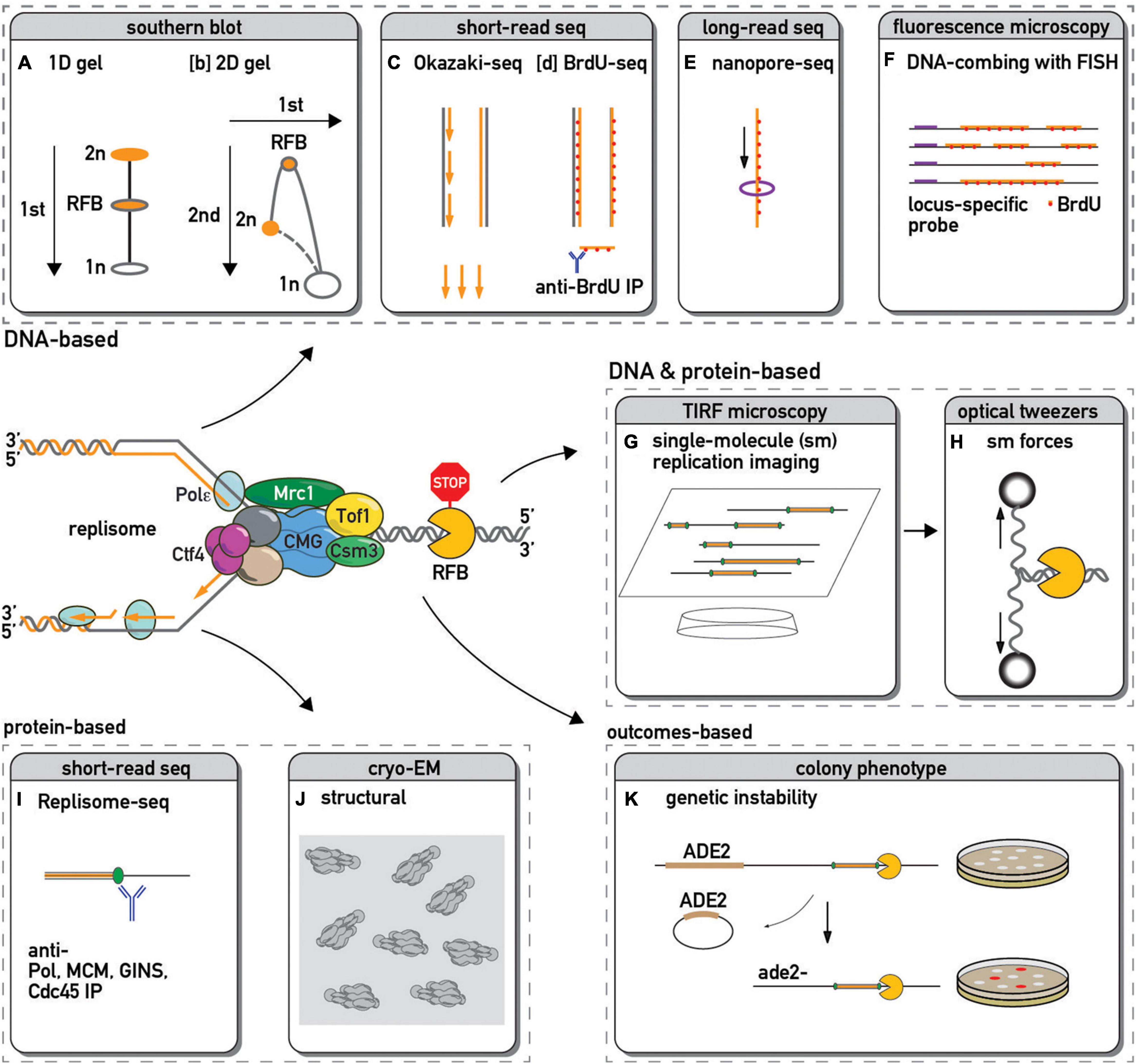
Frontiers Approaching Protein Barriers: Emerging Mechanisms of Replication Pausing in Eukaryotes

Single-molecule visualization of RecQ helicase reveals DNA melting, nucleation, and assembly are required for processive DNA unwinding
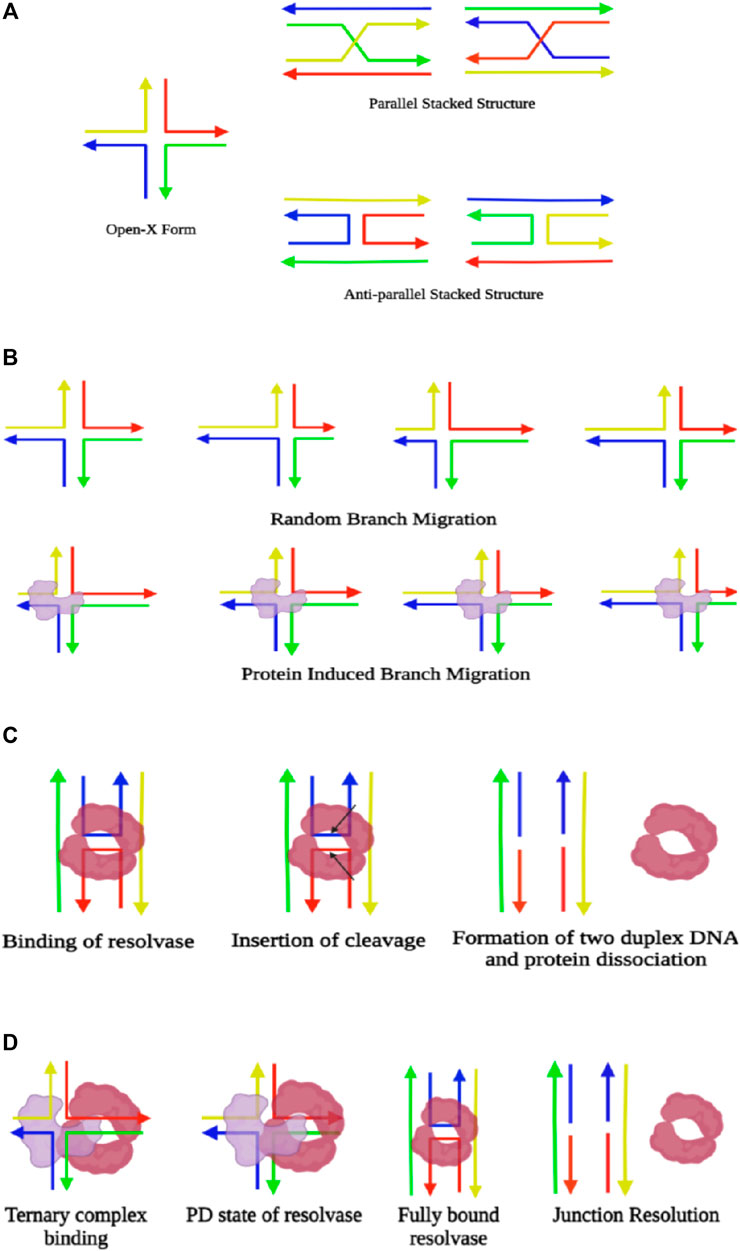
Frontiers Decoding the Structural Dynamics and Conformational Alternations of DNA Secondary Structures by Single-Molecule FRET Microspectroscopy
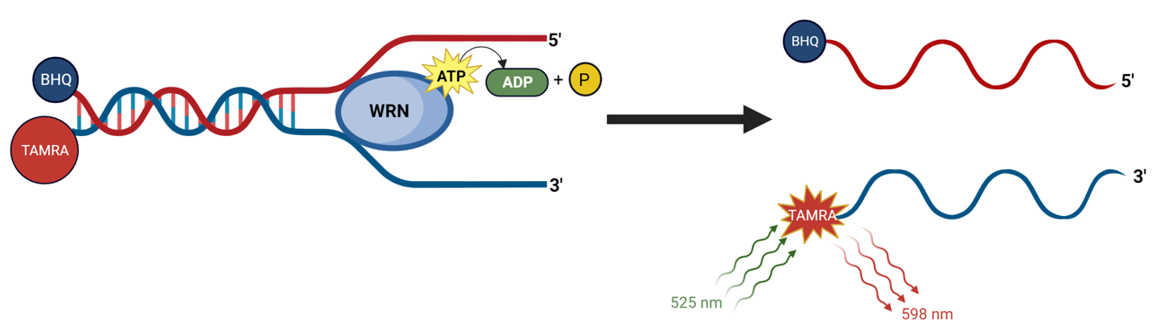
WRN Helicase Activity Assay Kit
Direct imaging of single UvrD helicase dynamics on long single-stranded DNA

DNA replication machinery: Insights from in vitro single-molecule approaches - Computational and Structural Biotechnology Journal
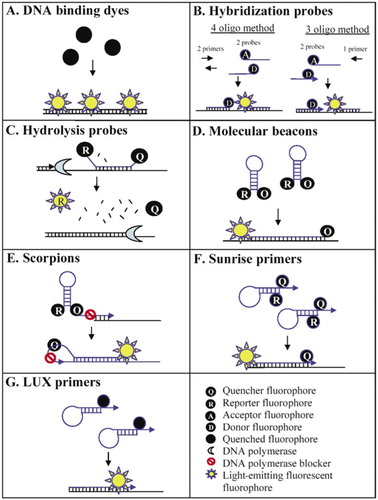
Real-time PCR for mRNA quantitation

Fluorescent Single-Stranded DNA Binding Protein as a Probe for Sensitive, Real-Time Assays of Helicase Activity - ScienceDirect

PDF] Fluorescence stopped-flow studies of single turnover kinetics of E.coli RecBCD helicase-catalyzed DNA unwinding.

PDF] Fluorescence stopped-flow studies of single turnover kinetics of E.coli RecBCD helicase-catalyzed DNA unwinding.
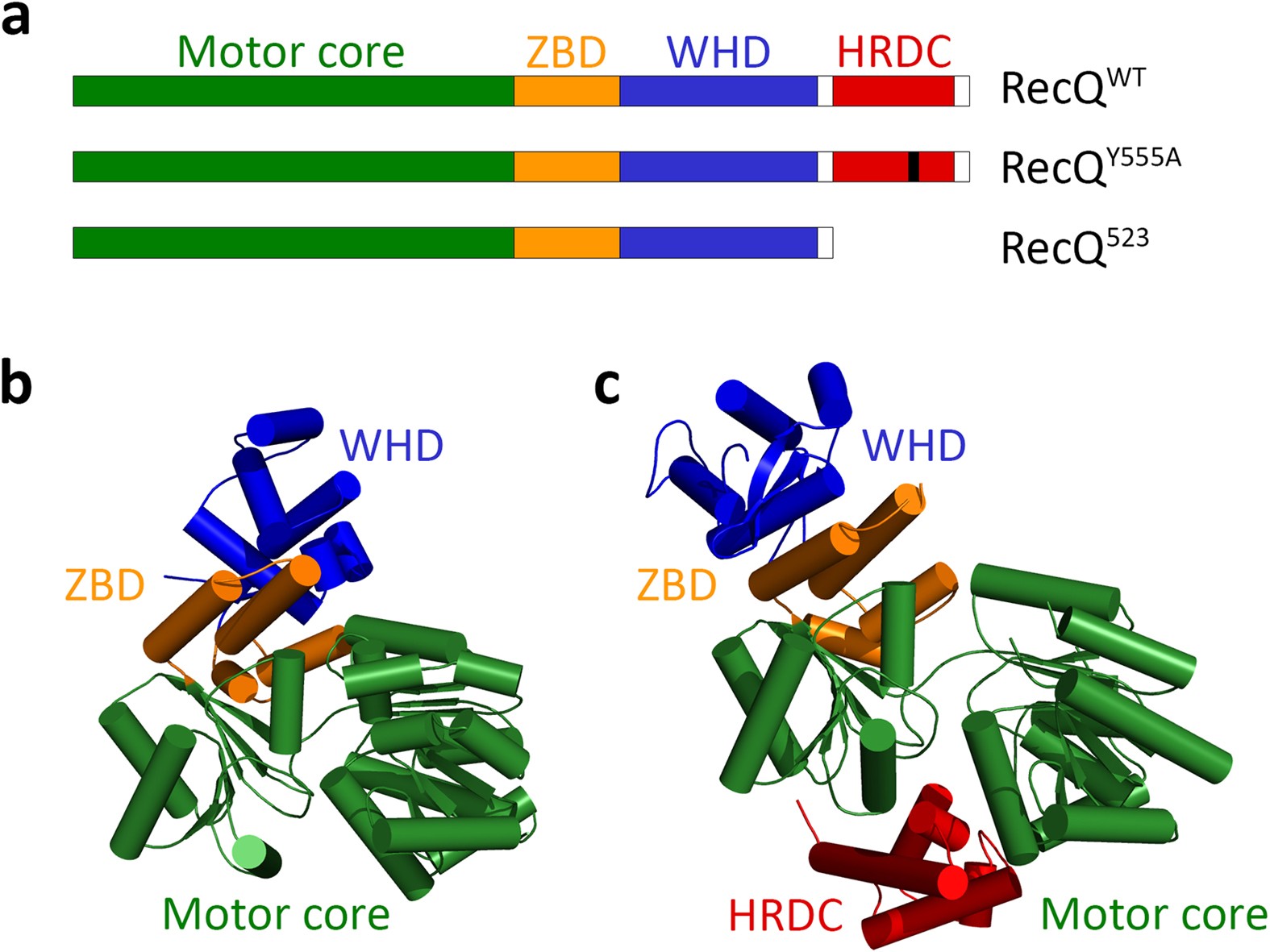
The HRDC domain of E. coli RecQ helicase controls single-stranded DNA translocation and double-stranded DNA unwinding rates without affecting mechanoenzymatic coupling

RNA‐Selective Small‐Molecule Ligands: Recent Advances in Live‐Cell Imaging and Drug Discovery - Chan - 2023 - ChemMedChem - Wiley Online Library
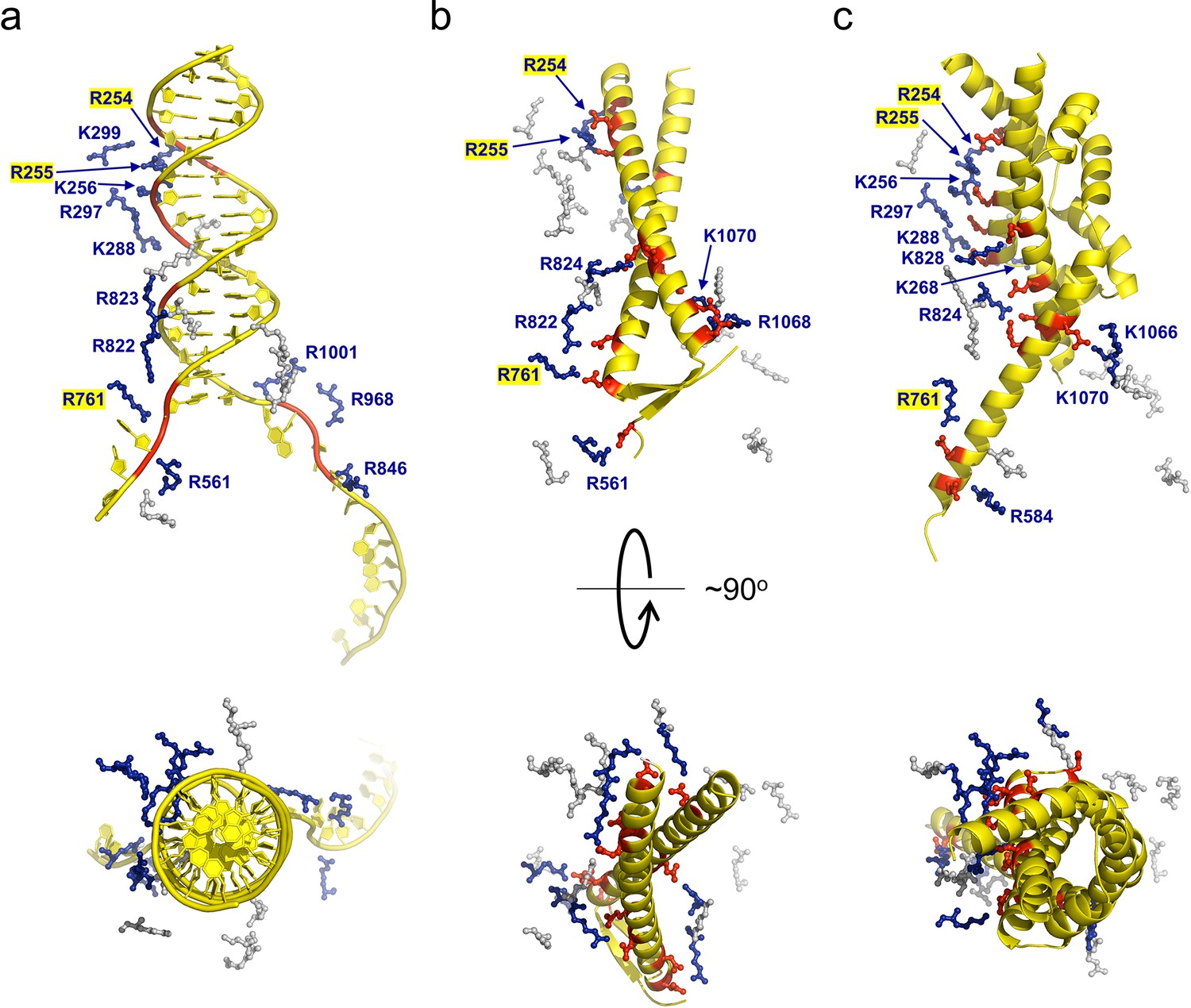
Structures of RecBCD in complex with phage-encoded inhibitor proteins reveal distinctive strategies for evasion of a bacterial immunity hub

The DNA replication initiation protein DnaD is recruited to a specific strand of the Bacillus subtilis chromosome origin
Recomendado para você
-
 Ghost of Tsushima The Tale of Ryuzo Walkthrough31 março 2025
Ghost of Tsushima The Tale of Ryuzo Walkthrough31 março 2025 -
 I am stranded on a desert island far from home. Please send help. - Creepypasta31 março 2025
I am stranded on a desert island far from home. Please send help. - Creepypasta31 março 2025 -
 It took me forever to find this, hoping it will help some of you. The Stranded Dead - Chapter 3 Gyozen's lost scroll : r/ghostoftsushima31 março 2025
It took me forever to find this, hoping it will help some of you. The Stranded Dead - Chapter 3 Gyozen's lost scroll : r/ghostoftsushima31 março 2025 -
 Australia says about 230 pilot whales stranded in Tasmania, half feared dead31 março 2025
Australia says about 230 pilot whales stranded in Tasmania, half feared dead31 março 2025 -
 The Dead Romantics: A GMA Book Club Pick by Poston, Ashley31 março 2025
The Dead Romantics: A GMA Book Club Pick by Poston, Ashley31 março 2025 -
 Researchers: Dolphins found dead were stranded during Sally31 março 2025
Researchers: Dolphins found dead were stranded during Sally31 março 2025 -
Indonesian volunteers save six beached whales31 março 2025
-
 67 (or Fewer) Horror Movies to Watch If You're Stranded on a Desert Island Like I Was - HubPages31 março 2025
67 (or Fewer) Horror Movies to Watch If You're Stranded on a Desert Island Like I Was - HubPages31 março 2025 -
 Morrowind Dunmer VRM at Valheim Nexus - Mods and community31 março 2025
Morrowind Dunmer VRM at Valheim Nexus - Mods and community31 março 2025 -
 Counting the dead in Iraq's Mosul city and other stories you may have missed this week31 março 2025
Counting the dead in Iraq's Mosul city and other stories you may have missed this week31 março 2025
você pode gostar
-
 Perfil - Roblox Cool avatars, Anime best friends, Create avatar free31 março 2025
Perfil - Roblox Cool avatars, Anime best friends, Create avatar free31 março 2025 -
 Half-Life: Alyx is not a retcon. It's a direct sequel to Episode 231 março 2025
Half-Life: Alyx is not a retcon. It's a direct sequel to Episode 231 março 2025 -
Gramados Virtuais Campeonatos de Futebol Virtual31 março 2025
-
 El gran Uruguay de las Copas del Mundo se comenzó a gestar en 192431 março 2025
El gran Uruguay de las Copas del Mundo se comenzó a gestar en 192431 março 2025 -
 N64 Game Rental: Tony Hawk's Pro Skater 2 – 1up Video Game Rentals31 março 2025
N64 Game Rental: Tony Hawk's Pro Skater 2 – 1up Video Game Rentals31 março 2025 -
 Powerball lottery jackpot at $441M; winning numbers drawing Wednesday - 6abc Philadelphia31 março 2025
Powerball lottery jackpot at $441M; winning numbers drawing Wednesday - 6abc Philadelphia31 março 2025 -
 Noobs Vs Zombies Realish weapon ideas31 março 2025
Noobs Vs Zombies Realish weapon ideas31 março 2025 -
 Sem Spoiler on X: Acaba de ser divulgada a capa de “Heavenly31 março 2025
Sem Spoiler on X: Acaba de ser divulgada a capa de “Heavenly31 março 2025 -
 Fotos en Club Atletico Atlanta - Sede Social - Deportes y ocio en31 março 2025
Fotos en Club Atletico Atlanta - Sede Social - Deportes y ocio en31 março 2025 -
 Desenhos Animados Desenhados à Mão E Clipart De Gato Amarelo PNG31 março 2025
Desenhos Animados Desenhados à Mão E Clipart De Gato Amarelo PNG31 março 2025
