Diagnostics and analysis of SARS-CoV-2: current status, recent
Por um escritor misterioso
Last updated 29 março 2025
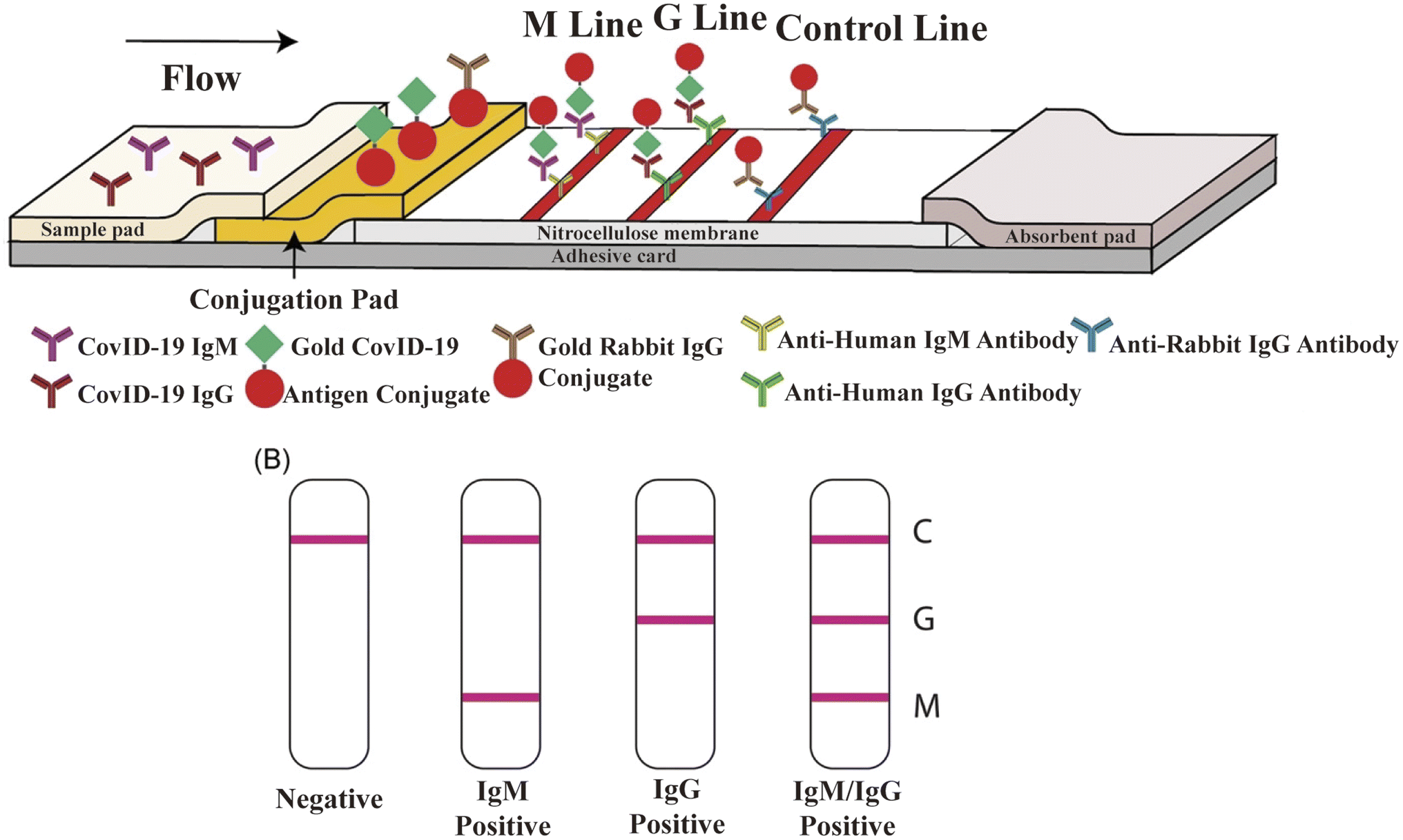

Two Years into the COVID-19 Pandemic: Lessons Learned

Coronavirus

Mild or Moderate Covid-19
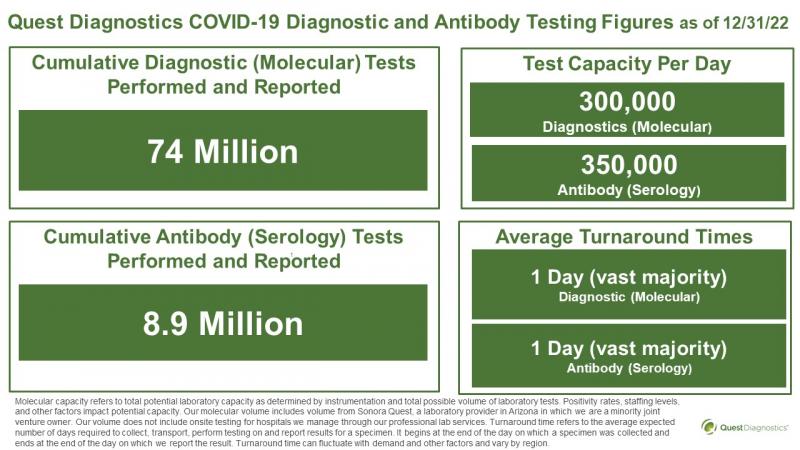
Quest Diagnostics Newsroom - News Releases
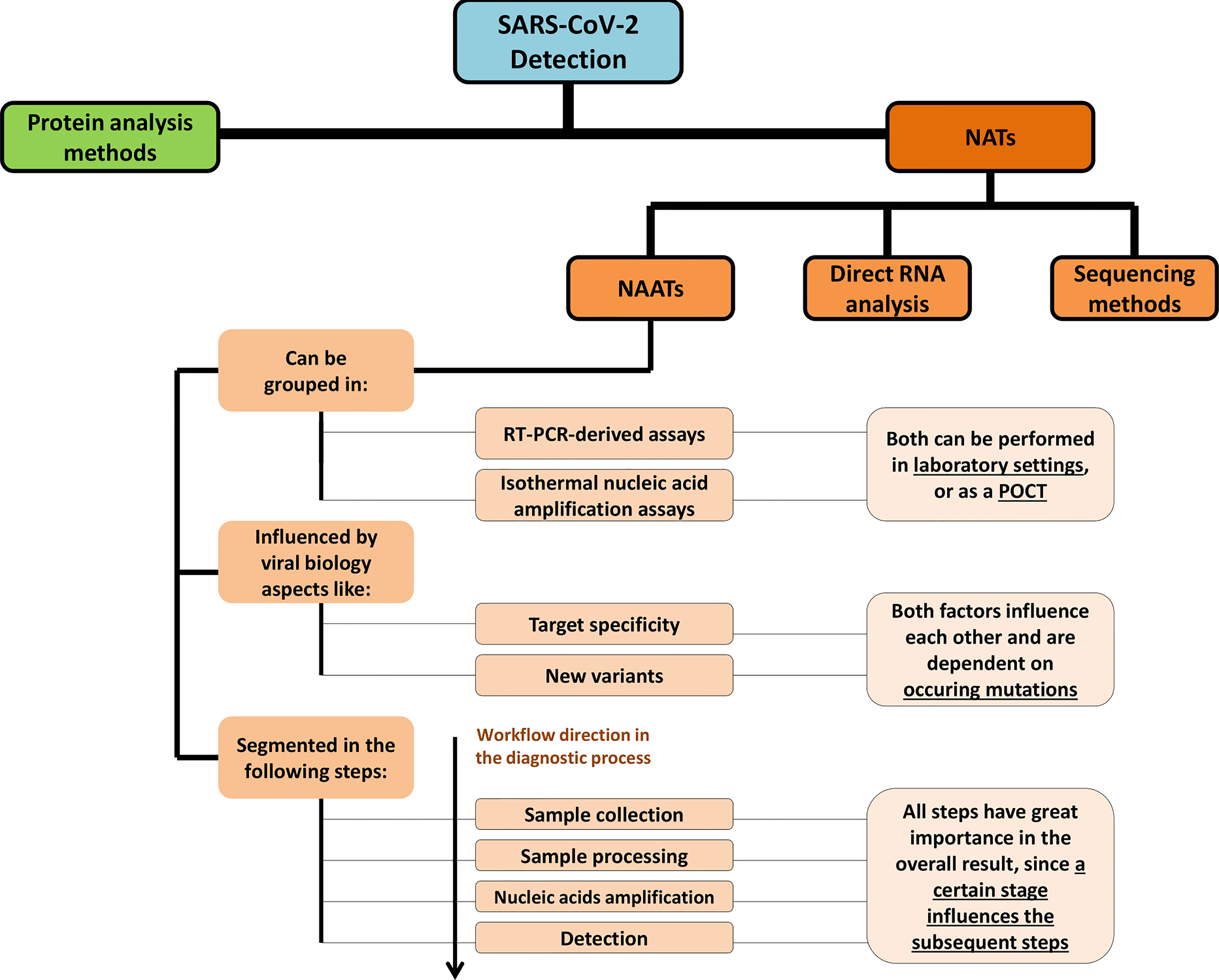
Frontiers SARS-CoV-2 Diagnostics Based on Nucleic Acids Amplification: From Fundamental Concepts to Applications and Beyond
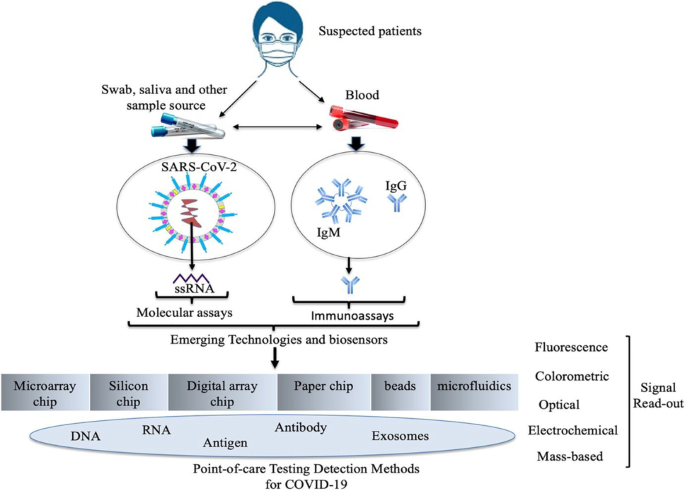
Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic
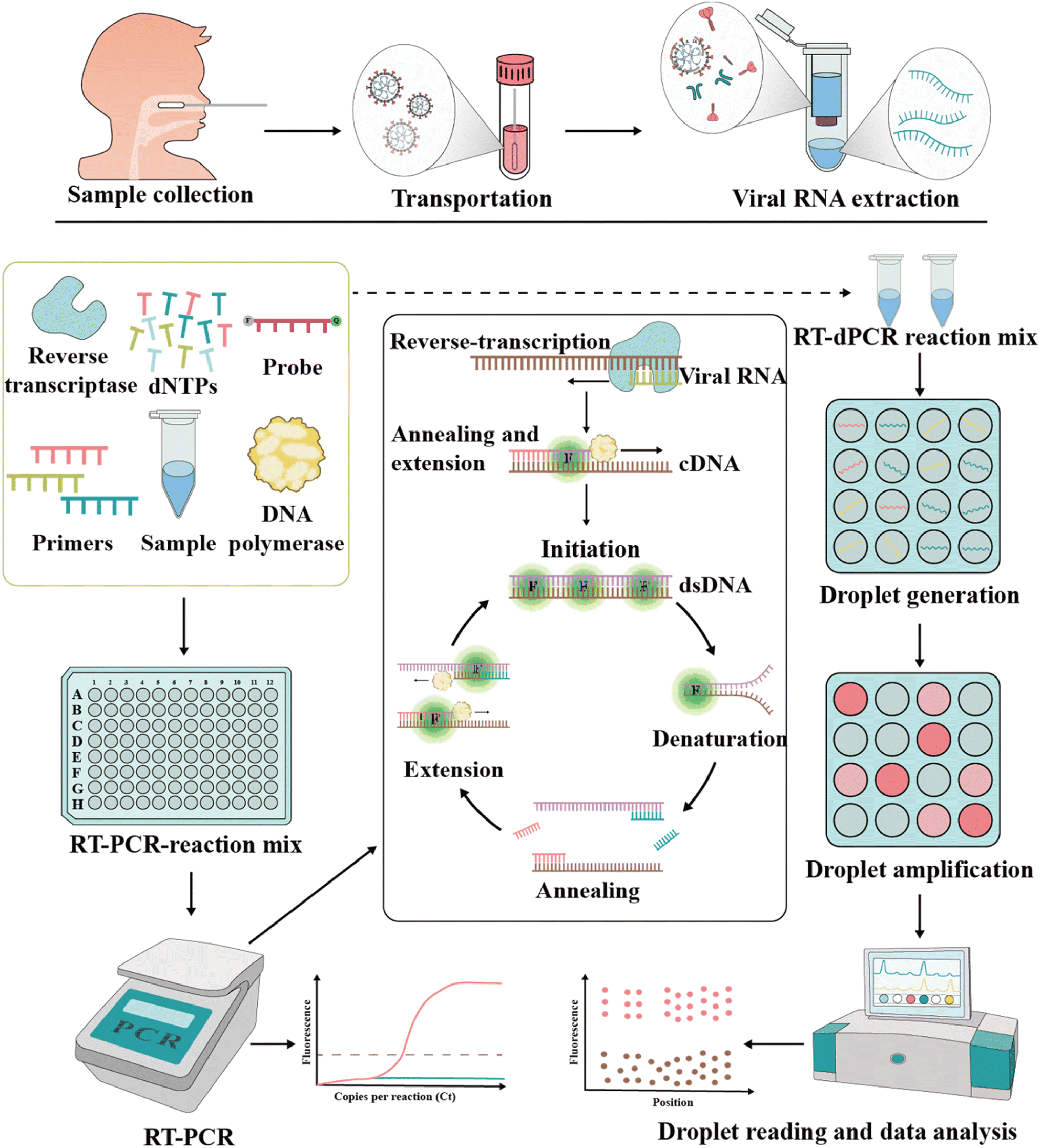
Diagnostics and analysis of SARS-CoV-2: current status, recent advances, challenges and perspectives - Chemical Science (RSC Publishing) DOI:10.1039/D2SC06665C
.png)
Xpert® Xpress SARS-CoV-2 - FDA Emergency Use Authorization

Emergence of SARS-CoV-2 B.1.1.7 Lineage — United States, December 29, 2020–January 12, 2021
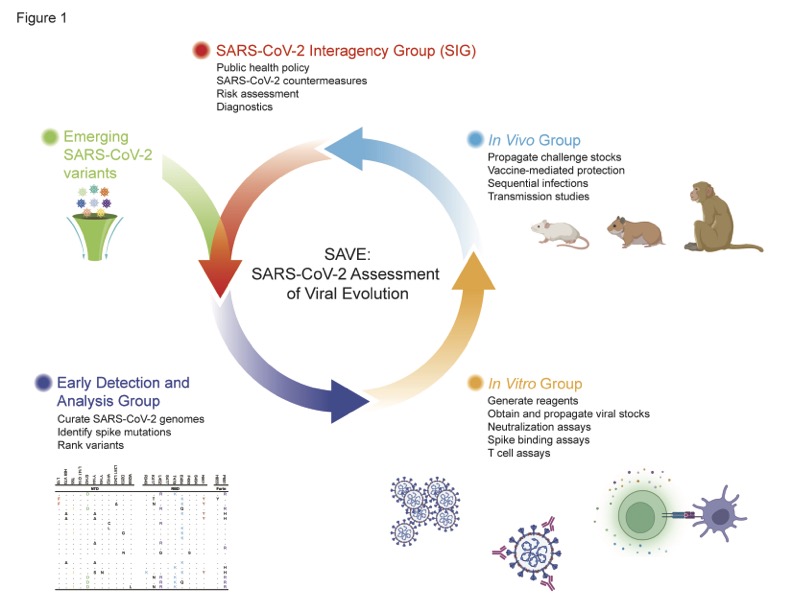
SAVE—NIAID's 'Avengers-Like' Research Program for Evolving Pathogens
Recomendado para você
-
 Shotgun King The Final Checkmate v1.37 – Skidrow & Reloaded Games29 março 2025
Shotgun King The Final Checkmate v1.37 – Skidrow & Reloaded Games29 março 2025 -
 Transcriptional and clonal characterization of B cell plasmablast29 março 2025
Transcriptional and clonal characterization of B cell plasmablast29 março 2025 -
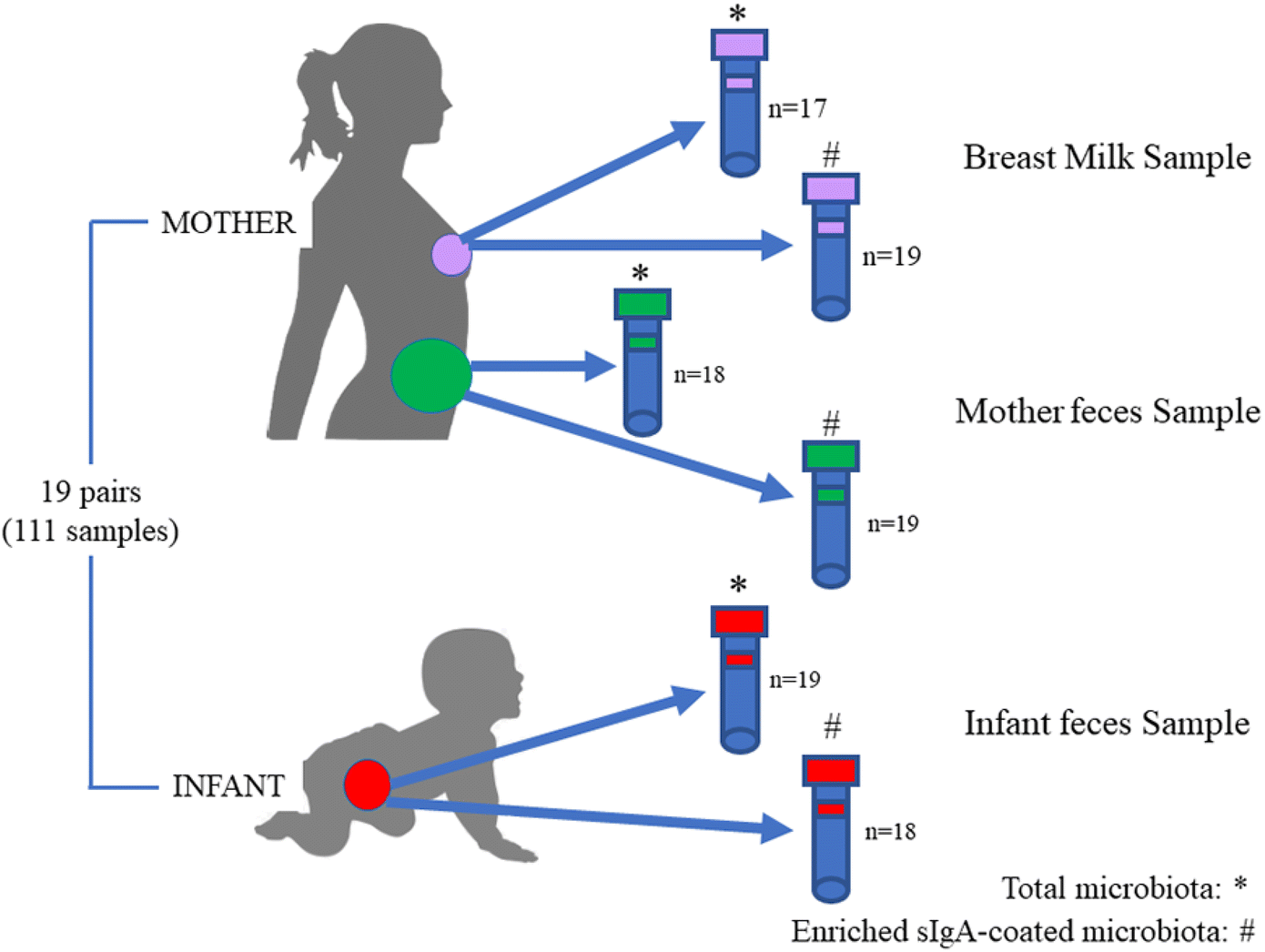 Widespread vertical transmission of secretory immunoglobulin A29 março 2025
Widespread vertical transmission of secretory immunoglobulin A29 março 2025 -
Competition between Serum IgG, IgM, and IgA Anti-Glycan Antibodies29 março 2025
-
 Expediting Antibody Discovery with a Cell and Bead Multiplexed29 março 2025
Expediting Antibody Discovery with a Cell and Bead Multiplexed29 março 2025 -
 Tertiary lymphoid structures generate and propagate anti-tumor29 março 2025
Tertiary lymphoid structures generate and propagate anti-tumor29 março 2025 -
 TheNightcapKing - Hobbyist, General Artist29 março 2025
TheNightcapKing - Hobbyist, General Artist29 março 2025 -
 Ill-Gotten Gains Update Parts One and Two - GTA 5 Guide - IGN29 março 2025
Ill-Gotten Gains Update Parts One and Two - GTA 5 Guide - IGN29 março 2025 -
 From structure to function – Ligand recognition by myeloid C-type29 março 2025
From structure to function – Ligand recognition by myeloid C-type29 março 2025 -
 Reading IGN's top 100 RPG list, when I noticed something odd29 março 2025
Reading IGN's top 100 RPG list, when I noticed something odd29 março 2025
você pode gostar
-
 Five Nights At Freddy's Is Poised To Become The Next Big Horror Sensation At The Box Office29 março 2025
Five Nights At Freddy's Is Poised To Become The Next Big Horror Sensation At The Box Office29 março 2025 -
 Desenholandia Colorindo Jogador de Futebol do Roblox Games Jogo Diversão29 março 2025
Desenholandia Colorindo Jogador de Futebol do Roblox Games Jogo Diversão29 março 2025 -
 Legendary Star-Lord (2014) #4, Comic Issues29 março 2025
Legendary Star-Lord (2014) #4, Comic Issues29 março 2025 -
 Cylindrical wood beads hand-sewn in a unique design over a metal frame. Mirror comes with metal29 março 2025
Cylindrical wood beads hand-sewn in a unique design over a metal frame. Mirror comes with metal29 março 2025 -
 According to urban dictionary on fleek means Zayn Malik29 março 2025
According to urban dictionary on fleek means Zayn Malik29 março 2025 -
 Pop! Games: Cuphead - Aeroplane Ms. Chalice29 março 2025
Pop! Games: Cuphead - Aeroplane Ms. Chalice29 março 2025 -
 Pokemon Figura Charizard Chama e Voo Com Pikachu - Sunny 3296 - Fabrica da Alegria29 março 2025
Pokemon Figura Charizard Chama e Voo Com Pikachu - Sunny 3296 - Fabrica da Alegria29 março 2025 -
 How to change your roblox font to the minecraft font29 março 2025
How to change your roblox font to the minecraft font29 março 2025 -
 3D Printed Roblox Robux 7 Coins29 março 2025
3D Printed Roblox Robux 7 Coins29 março 2025 -
 Raichu GX Official Pokemon Cards - GX, VMAX, EX or V29 março 2025
Raichu GX Official Pokemon Cards - GX, VMAX, EX or V29 março 2025