Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial - The Lancet
Por um escritor misterioso
Last updated 30 março 2025


Ischaemic stroke despite antiplatelet therapy: Causes and outcomes

Clinical Evaluation of Factor XIa Inhibitor Drugs: JACC Review

Frequency and Patterns of Brain Infarction in Patients With

Safety of the oral factor XIa inhibitor asundexian compared with

Safety and efficacy of factor XIa inhibition with milvexian for
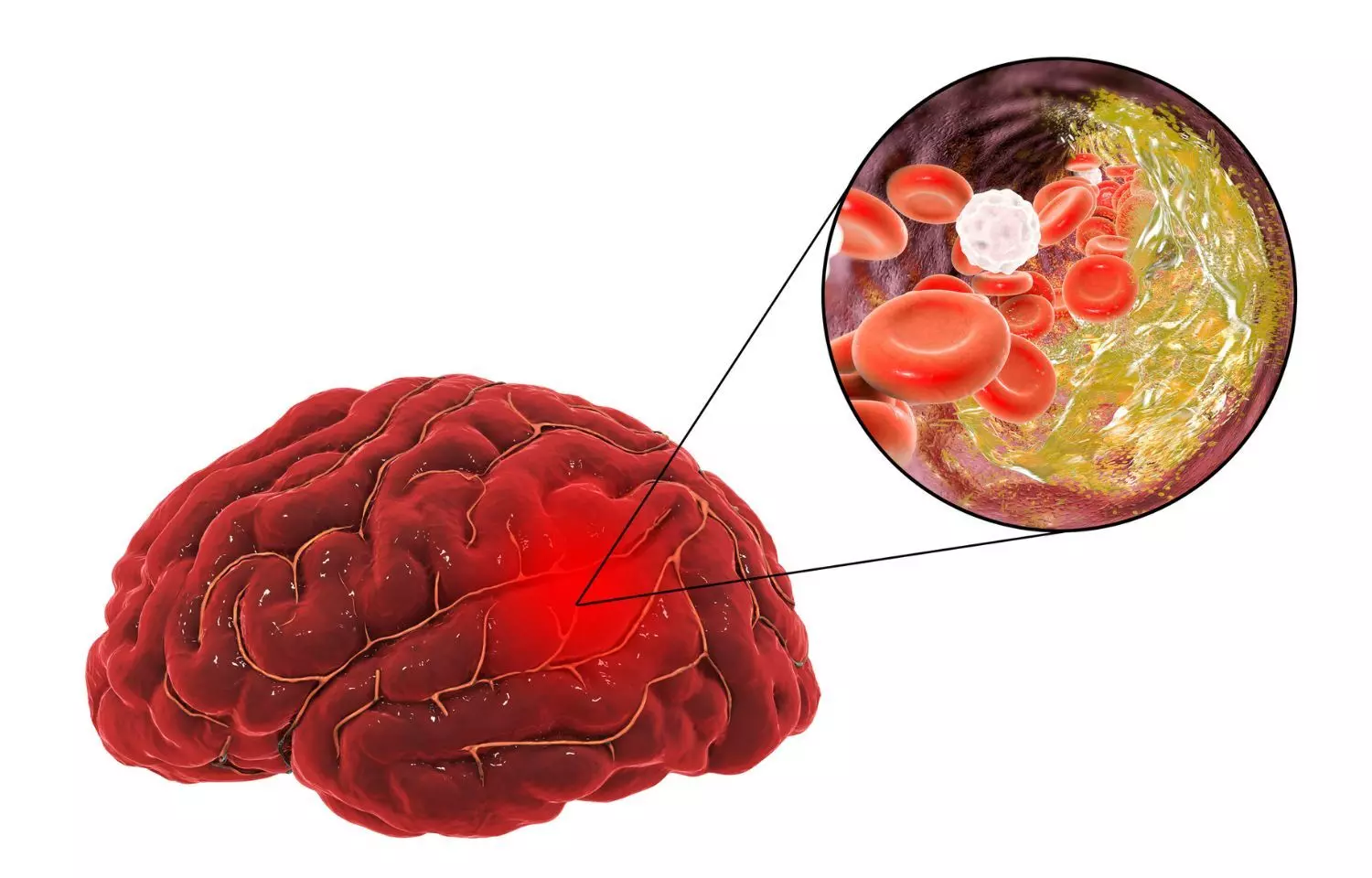
New anticoagulant asundexian prevents recurrent ischaemic stroke

7. Inhibición del Factor XIa con asundexian tras ictus isquémico

Asundexian (BAY-2433334), CAS 2064121-65-7
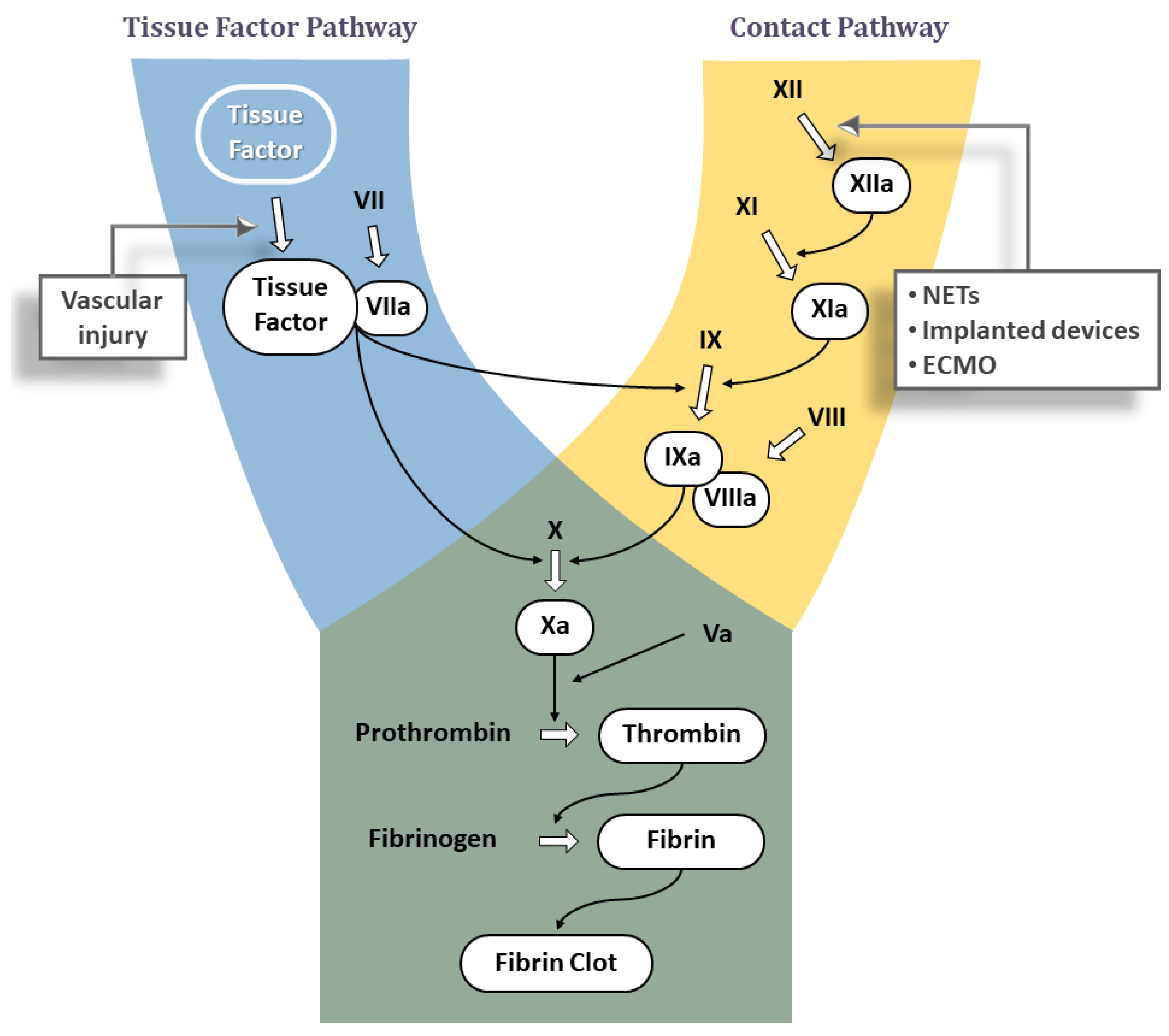
JCDD, Free Full-Text

Design and Preclinical Characterization Program toward Asundexian

Design and Preclinical Characterization Program toward Asundexian

Anticoagulation

PDF) Pharmacological targets of Asundexian relevant to its
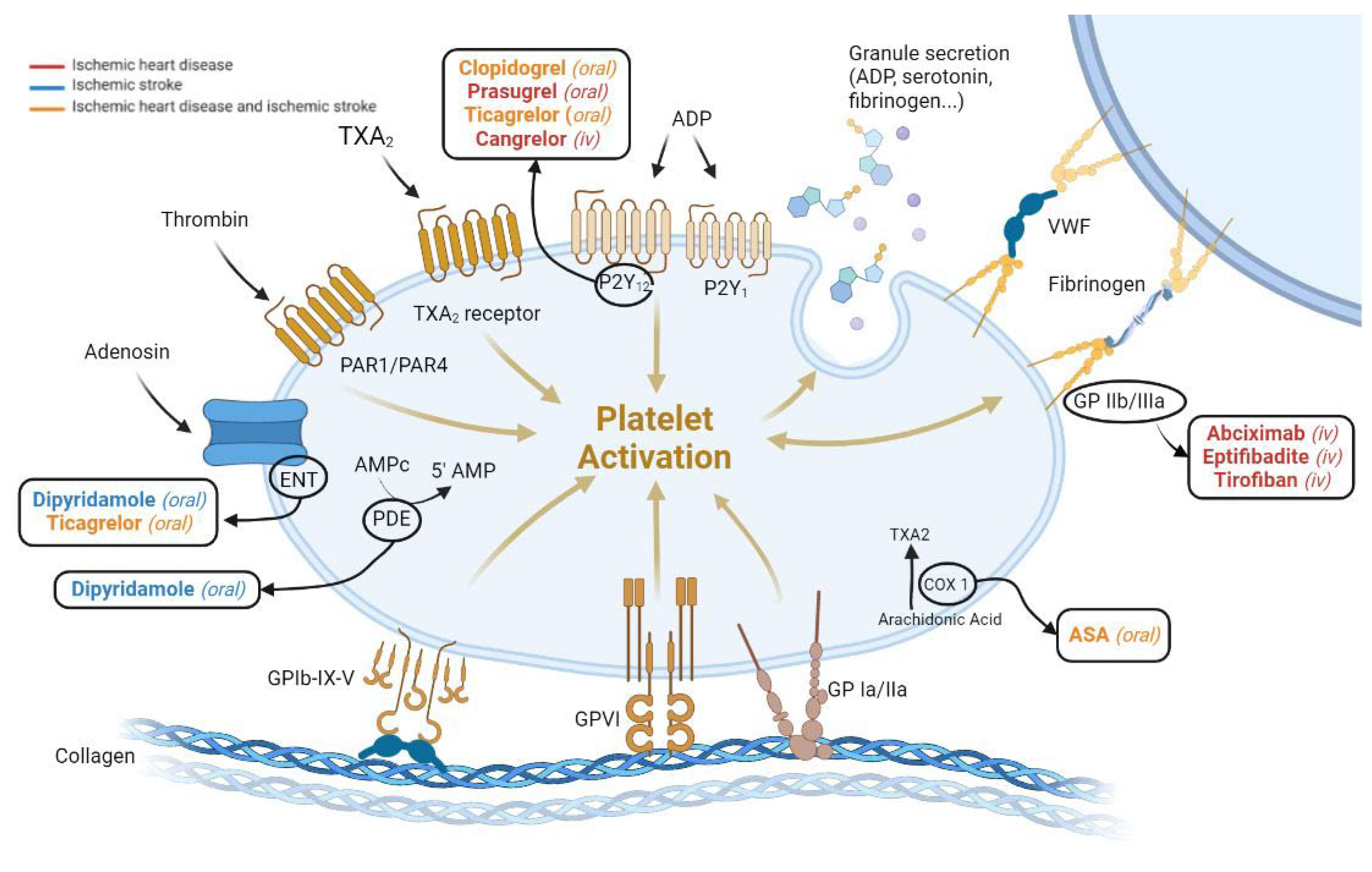
JCDD, Free Full-Text
Recomendado para você
-
 BRAIN TEST NÍVEL 372 EM PORTUGUÊS30 março 2025
BRAIN TEST NÍVEL 372 EM PORTUGUÊS30 março 2025 -
 Он хочет быть выше. 372 уровень Brain Test30 março 2025
Он хочет быть выше. 372 уровень Brain Test30 março 2025 -
 Brain Test Level 372 He wants big muscles Walkthrough30 março 2025
Brain Test Level 372 He wants big muscles Walkthrough30 março 2025 -
 Close this dialog30 março 2025
Close this dialog30 março 2025 -
 Western blots show p65 antibodies that passed the test of specificity30 março 2025
Western blots show p65 antibodies that passed the test of specificity30 março 2025 -
 Easy Game Brain Test Level 372 Finish shopping.30 março 2025
Easy Game Brain Test Level 372 Finish shopping.30 março 2025 -
Optical challenge: A Sherlock Holmes-like mind can find the hidden potato in 11 seconds! - alro news30 março 2025
-
Brain Sciences Center - News30 março 2025
-
 Grain Brain: The Surprising Truth by Perlmutter MD, David30 março 2025
Grain Brain: The Surprising Truth by Perlmutter MD, David30 março 2025 -
 Lactate Attenuates Synaptic Transmission and Affects Brain Rhythms Featuring High Energy Expenditure - ScienceDirect30 março 2025
Lactate Attenuates Synaptic Transmission and Affects Brain Rhythms Featuring High Energy Expenditure - ScienceDirect30 março 2025
você pode gostar
-
 200 melhores nomes para guildas - Nomes Criativos30 março 2025
200 melhores nomes para guildas - Nomes Criativos30 março 2025 -
 SPACETREK66 - DVD TRANSFORMERS 5 - O ULTIMO CAVALEIRO30 março 2025
SPACETREK66 - DVD TRANSFORMERS 5 - O ULTIMO CAVALEIRO30 março 2025 -
 My Next Life as a Villainess: All Routes Lead to Doom! - Pirates30 março 2025
My Next Life as a Villainess: All Routes Lead to Doom! - Pirates30 março 2025 -
 Boy Running Sticker - Boy Running - Discover & Share GIFs30 março 2025
Boy Running Sticker - Boy Running - Discover & Share GIFs30 março 2025 -
 Download Cut the Rope MOD APK v3.34.0 for Android30 março 2025
Download Cut the Rope MOD APK v3.34.0 for Android30 março 2025 -
 Where to Go in a Whole Other World?:Isekai Ni30 março 2025
Where to Go in a Whole Other World?:Isekai Ni30 março 2025 -
 Tales of Demons and Gods - Chapter 116 Battle At The Corn Field30 março 2025
Tales of Demons and Gods - Chapter 116 Battle At The Corn Field30 março 2025 -
 Uma pergunta por dia: 365 perguntas - 5 anos - 1.825 respostas30 março 2025
Uma pergunta por dia: 365 perguntas - 5 anos - 1.825 respostas30 março 2025 -
 Brasileirão Table: All Open with 3 Rounds to Go - Calcio Deal30 março 2025
Brasileirão Table: All Open with 3 Rounds to Go - Calcio Deal30 março 2025 -
 Nerf Roblox MM2: Shark Seeker Blaster30 março 2025
Nerf Roblox MM2: Shark Seeker Blaster30 março 2025