Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 28 março 2025

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.
Target Product Profiling & Drug Safety Assessment - SciLifeLab

Why 90% of clinical drug development fails and how to improve it

Principles of early drug discovery - Hughes - 2011 - British

Which Human Metabolites Have We MIST? Retrospective Analysis

Drug development – The four phases - BioStock

PDF) Investigative safety strategies to improve success in drug

Label-free drug discovery strategy. Combining computational

Early Safety Assessment - Drug Discovery and Development Based on

Preclinical Working Group National Institutes of Health (NIH)
Recomendado para você
-
 BRAIN TEST LEVEL 411 WALK THROUGH WITH COMMENTARY28 março 2025
BRAIN TEST LEVEL 411 WALK THROUGH WITH COMMENTARY28 março 2025 -
 Lengkap Ada Video, Brain Test Level 411 Sang Ksatria Harus28 março 2025
Lengkap Ada Video, Brain Test Level 411 Sang Ksatria Harus28 março 2025 -
 brain test nível 41128 março 2025
brain test nível 41128 março 2025 -
Death Incoming (411) #Android #Game #gameplay #gaming #apk #fat28 março 2025
-
 411 Adrenal Insufficiency with Dr. Atil Kargi - The Curbsiders28 março 2025
411 Adrenal Insufficiency with Dr. Atil Kargi - The Curbsiders28 março 2025 -
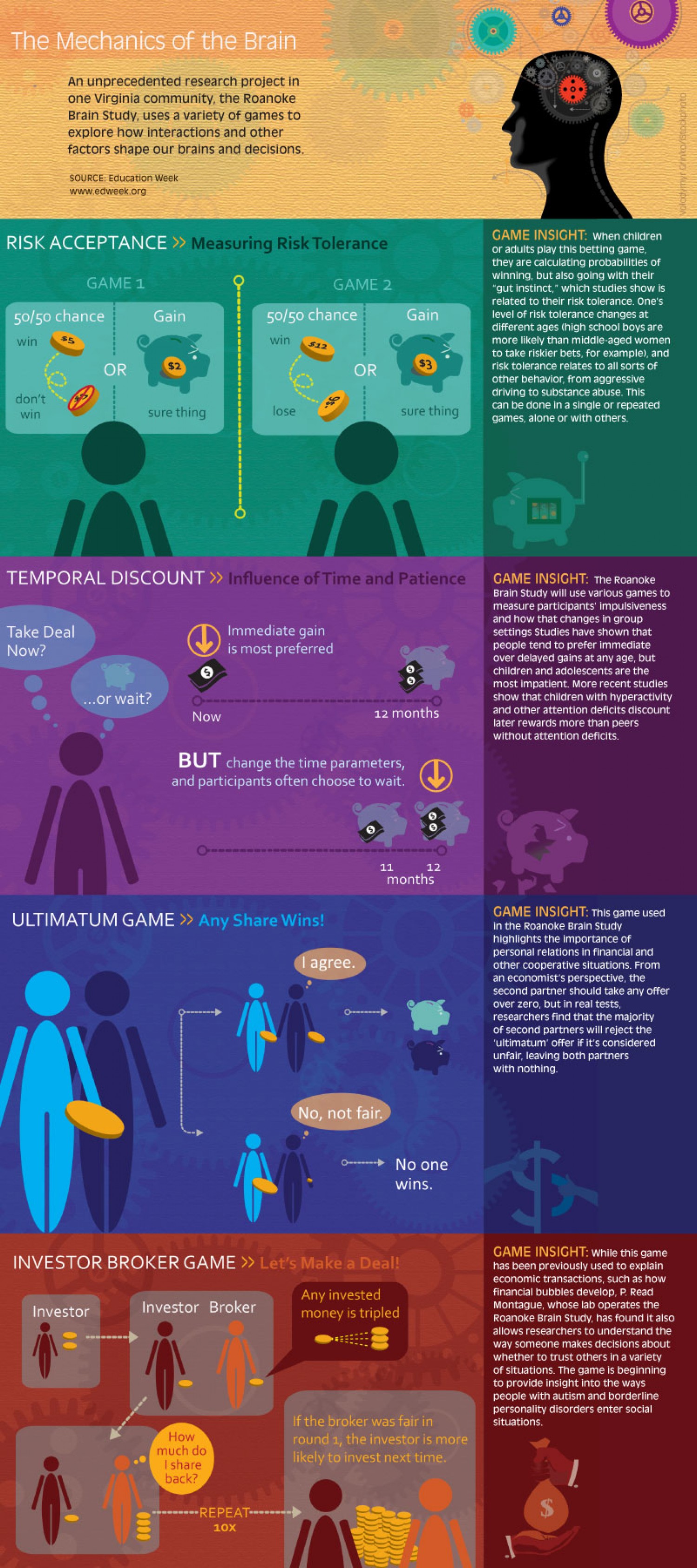 The Mechanics of the Brain (Infographic)28 março 2025
The Mechanics of the Brain (Infographic)28 março 2025 -
 Premium Vector Ent doctor scientist examine inflamed throat28 março 2025
Premium Vector Ent doctor scientist examine inflamed throat28 março 2025 -
 EJOYFL Brain Teaser Puzzle Unlock Interlock Puzzle IQ28 março 2025
EJOYFL Brain Teaser Puzzle Unlock Interlock Puzzle IQ28 março 2025 -
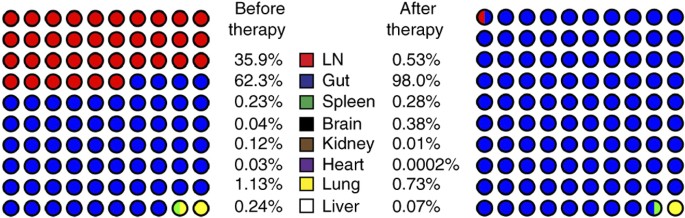 Defining total-body AIDS-virus burden with implications for28 março 2025
Defining total-body AIDS-virus burden with implications for28 março 2025 -
 √ Brain Test Level 351, 352, 353, 354, 355, 356, 357, 358,359,36028 março 2025
√ Brain Test Level 351, 352, 353, 354, 355, 356, 357, 358,359,36028 março 2025
você pode gostar
-
/media/movies/covers/2021/03/MV5BMWE1MTRhOTItOWU5Ni00YzEyLTlkZmItMTY5ZjI4MWMzNmExXkEyXkFqcGdeQX_D0ghCAd.jpg) Labirinto do Terror - 20 de Outubro de 202228 março 2025
Labirinto do Terror - 20 de Outubro de 202228 março 2025 -
 Muneeb on X: The Christmas Update for Catalog Avatar Creator is28 março 2025
Muneeb on X: The Christmas Update for Catalog Avatar Creator is28 março 2025 -
 Cantora Lorde escolherá as músicas da trilha de Jogos Vorazes: A28 março 2025
Cantora Lorde escolherá as músicas da trilha de Jogos Vorazes: A28 março 2025 -
 Retro World Expo ⋆ Hartford Has It28 março 2025
Retro World Expo ⋆ Hartford Has It28 março 2025 -
 A Plague Tale: Innocence – Things You Need To Know About The Story28 março 2025
A Plague Tale: Innocence – Things You Need To Know About The Story28 março 2025 -
 清水茜「はたらく細胞」が次号シリウスで完結、今号は「新型コロナウイルス編」 - コミックナタリー28 março 2025
清水茜「はたらく細胞」が次号シリウスで完結、今号は「新型コロナウイルス編」 - コミックナタリー28 março 2025 -
 Brown (A) vs. Atlanta - TyC Sports28 março 2025
Brown (A) vs. Atlanta - TyC Sports28 março 2025 -
 Pokemon Soul Silver Layout by KojiroBlade on DeviantArt28 março 2025
Pokemon Soul Silver Layout by KojiroBlade on DeviantArt28 março 2025 -
Mobile Hack Codes – Apps no Google Play28 março 2025
-
movies/reddit.md at master · labnol/movies · GitHub28 março 2025
