ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Last updated 03 abril 2025


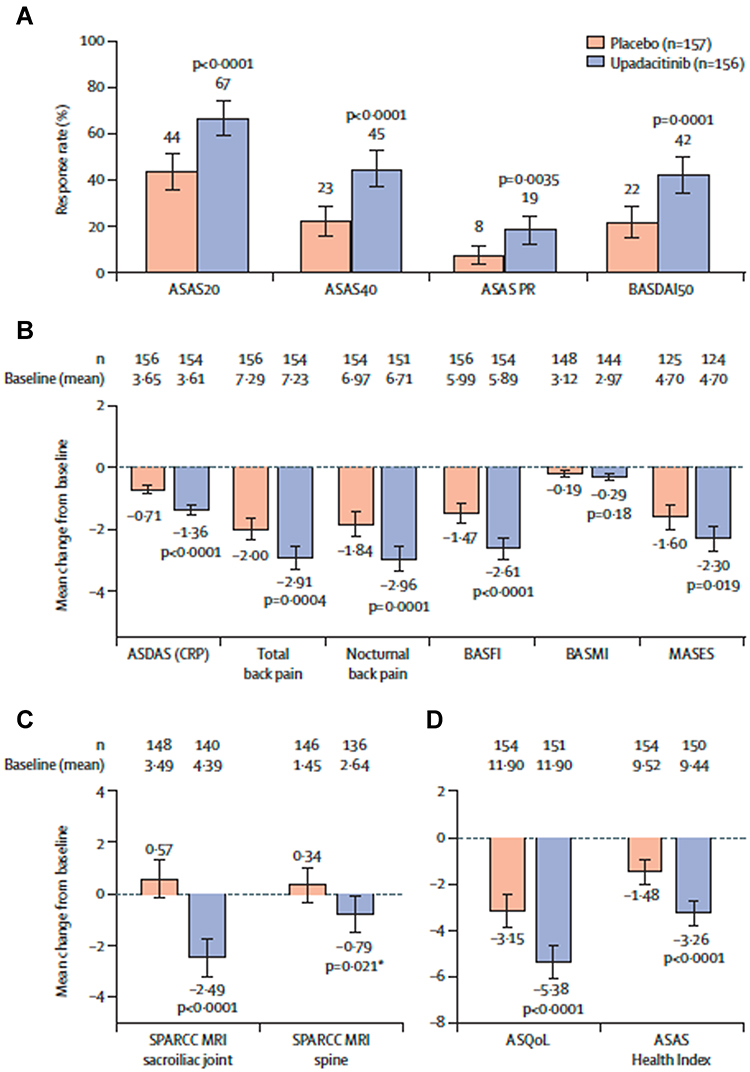
Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial

Disease Control Data, Ankylosing Spondylitis
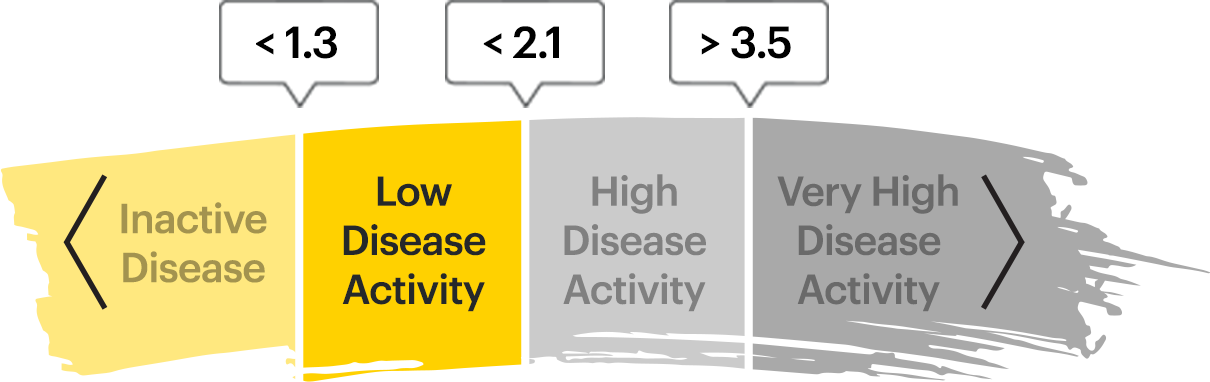
Disease Control Data, Ankylosing Spondylitis

PDF) Treatment response and drug retention rates in 24 195 biologic-naïve patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration
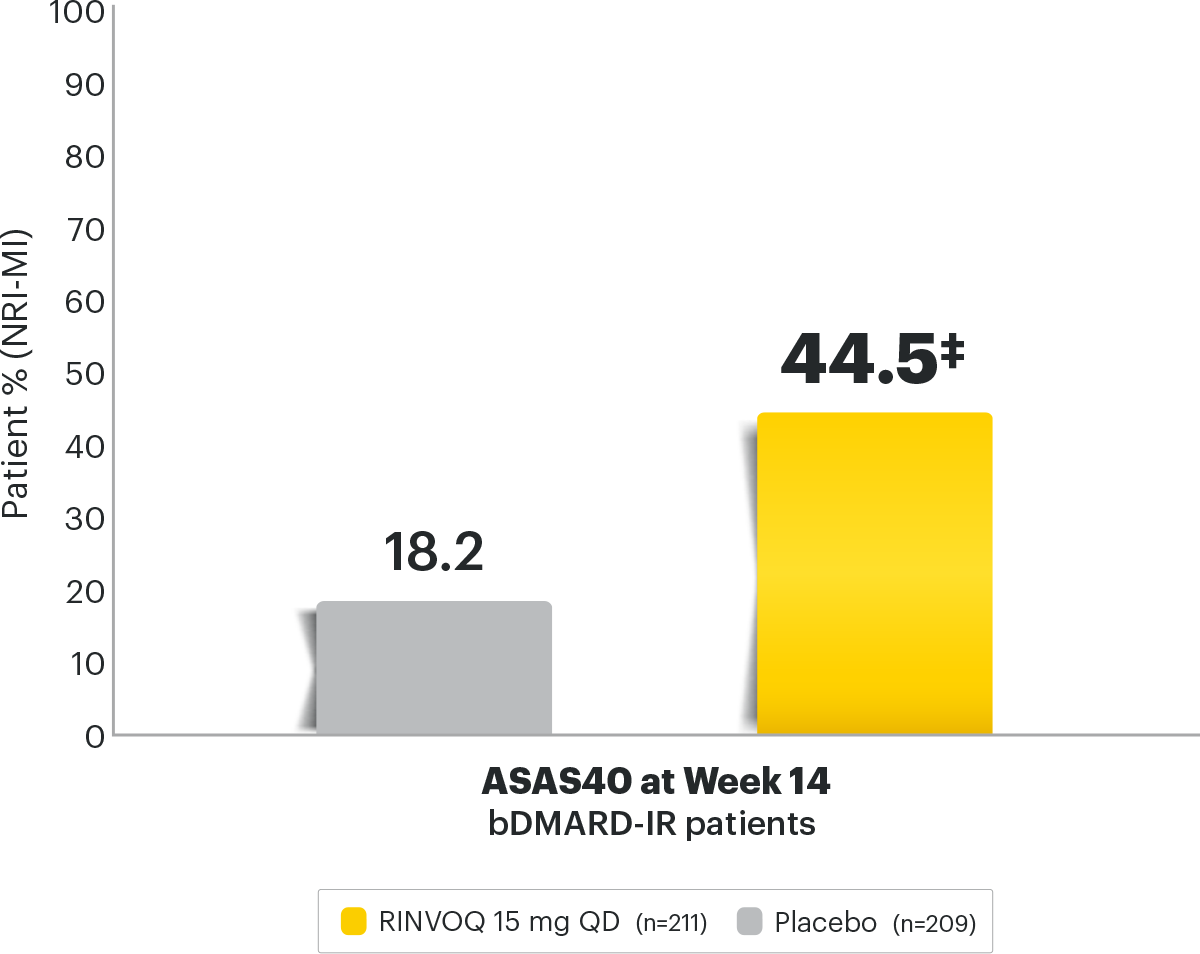
Axial Spondyloarthritis RINVOQ® (upadacitinib)

Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study
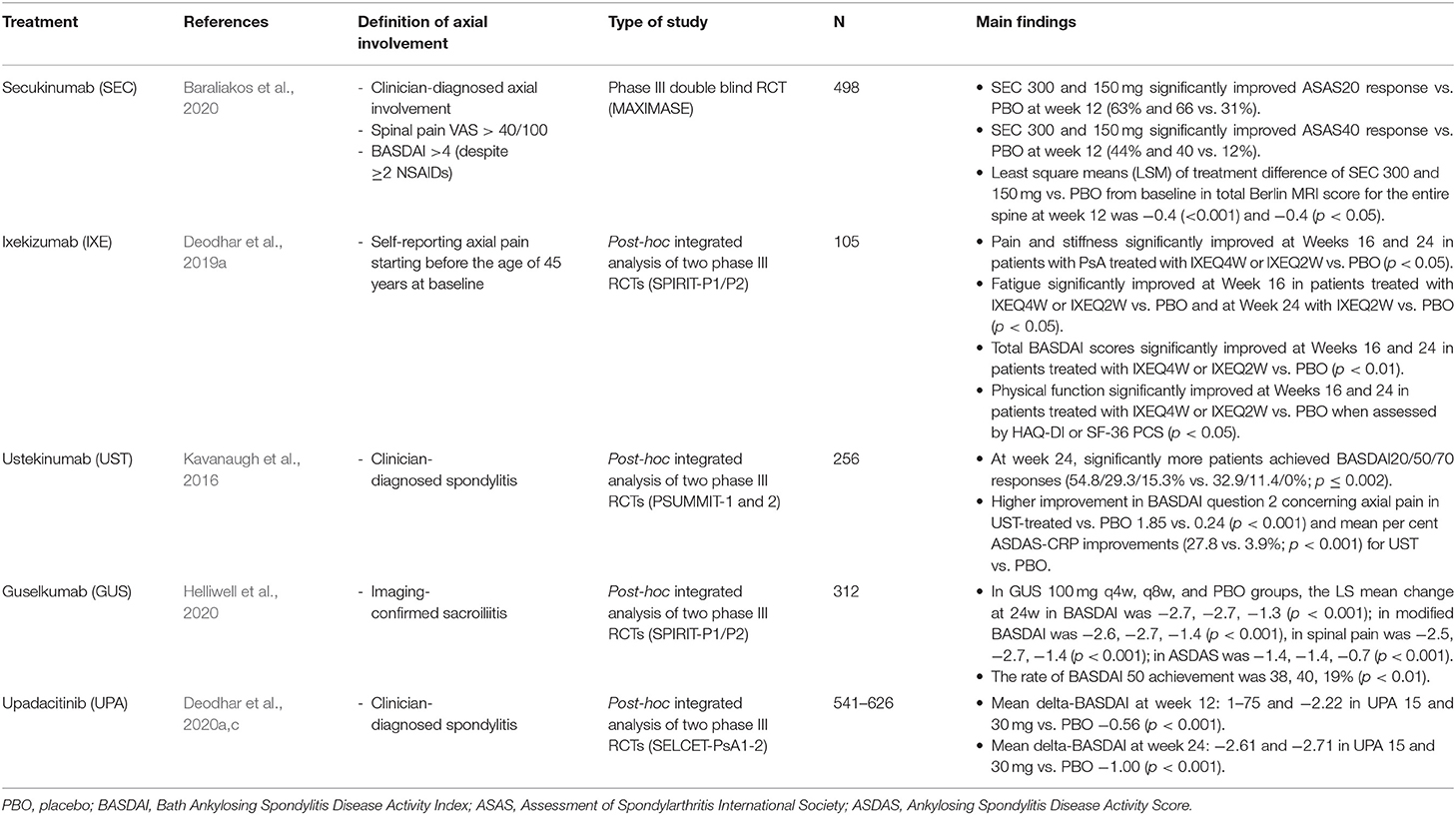
Frontiers Targeted Therapies in Axial Psoriatic Arthritis
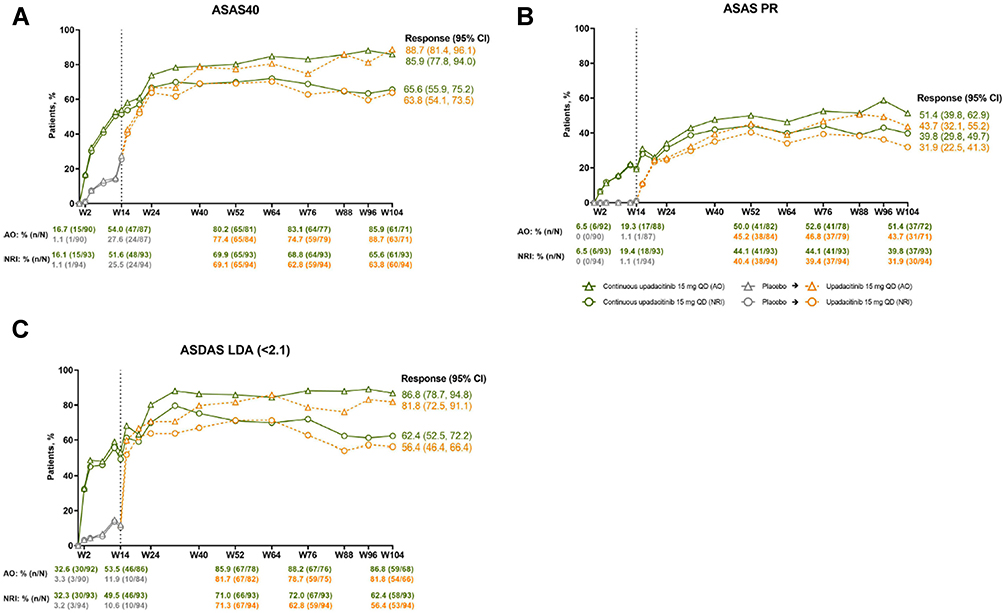
Management of axial spondyloarthritis
Management of axial spondyloarthritis
Recomendado para você
-
 Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores03 abril 2025
Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores03 abril 2025 -
ASAS_APP Did you know that ASAS has an app to facilitate the calculation of ASDAS? – click on this link to find out - ASAS - Assessment of SpondyloArthritis international Society03 abril 2025
-
![Scoring of disease activity using BASDAI and ASDAS method in ankylosing spondylitis].](https://d3i71xaburhd42.cloudfront.net/78fc15e3e8d403a79b972c5153e6c117273eaaed/4-Table4-1.png) Scoring of disease activity using BASDAI and ASDAS method in ankylosing spondylitis].03 abril 2025
Scoring of disease activity using BASDAI and ASDAS method in ankylosing spondylitis].03 abril 2025 -
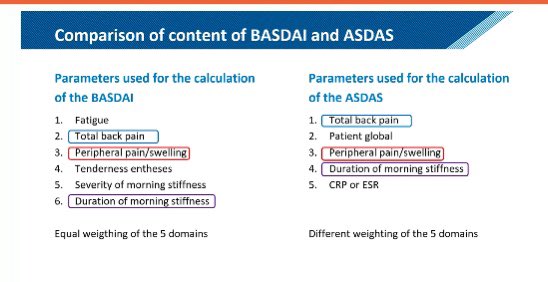 SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X03 abril 2025
SMC Arthritis Forum/Dr.Hanady Manasfi on X: Comparison #BASDAI and #ASDAS #spondyloarthtopathy #EULAR2020 / X03 abril 2025 -
 Mean Ankylosing Spondylitis Disease Activity Score with C‐reactive03 abril 2025
Mean Ankylosing Spondylitis Disease Activity Score with C‐reactive03 abril 2025 -
 Current Local Time in Asdas, Yemen03 abril 2025
Current Local Time in Asdas, Yemen03 abril 2025 -
 ASDAS Download Scientific Diagram03 abril 2025
ASDAS Download Scientific Diagram03 abril 2025 -
Asdas Svg Png Icon Free Download (#77064)03 abril 2025
-
 Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online03 abril 2025
Asda reveals real reason they use self-checkout cameras and it's not about data - Mirror Online03 abril 2025 -
 Asda - Wikipedia03 abril 2025
Asda - Wikipedia03 abril 2025
você pode gostar
-
 comfortably ugly blobfish03 abril 2025
comfortably ugly blobfish03 abril 2025 -
 RealRTCW - German Voice Pack on Steam03 abril 2025
RealRTCW - German Voice Pack on Steam03 abril 2025 -
 Call of Duty: Modern Warfare II - Task Force Intel03 abril 2025
Call of Duty: Modern Warfare II - Task Force Intel03 abril 2025 -
 Multicolor Shrinath Art Gallery Brass Chess Board Set03 abril 2025
Multicolor Shrinath Art Gallery Brass Chess Board Set03 abril 2025 -
 Fate/Stay Night: Heaven's Feel III. Spring Song Dominates The Box Office In Opening Weekend - Anime Corner03 abril 2025
Fate/Stay Night: Heaven's Feel III. Spring Song Dominates The Box Office In Opening Weekend - Anime Corner03 abril 2025 -
 Parauapebas: Polícia Civil apreende equipamentos de informática e fecha sites de transmissão de jogos e tv piratas – Jornal A Noticia03 abril 2025
Parauapebas: Polícia Civil apreende equipamentos de informática e fecha sites de transmissão de jogos e tv piratas – Jornal A Noticia03 abril 2025 -
 Árvore de Natal e presentes: saiba de onde vêm essas tradições natalinas03 abril 2025
Árvore de Natal e presentes: saiba de onde vêm essas tradições natalinas03 abril 2025 -
 My Hero Academia Confirms Season 4 Broadcast Date!, Anime News03 abril 2025
My Hero Academia Confirms Season 4 Broadcast Date!, Anime News03 abril 2025 -
 kurousagi, kasukabe you, kudou asuka, and sakamaki izayoi (mondaiji-tachi ga isekai kara kuru sou desu yo?) drawn by honda_yoshino03 abril 2025
kurousagi, kasukabe you, kudou asuka, and sakamaki izayoi (mondaiji-tachi ga isekai kara kuru sou desu yo?) drawn by honda_yoshino03 abril 2025 -
Steam Workshop::Mega Gengar Player model and NPC Pokemon XY Shiny mega ganagar03 abril 2025

